Osmotic Regulation
Osmoreceptors in the hypothalamus continuously monitor plasma osmolarity. Increased osmolarity stimulates ADH release, promoting water reabsorption in renal collecting ducts. This increases plasma volume and dilutes blood osmolarity back toward normal. Decreased osmolarity suppresses ADH release, reducing collecting duct water permeability and allowing water excretion as dilute urine. The osmotic threshold for ADH secretion is approximately 285 mOsm/kg; minimal ADH secretion occurs below this threshold, and maximal secretion occurs at osmolarity above 300 mOsm/kg.
Volume Regulation
Baroreceptors in carotid sinuses and aortic arch also regulate ADH. Significant blood volume decreases stimulate ADH release despite decreased plasma osmolarity, overriding osmotic inhibition. This volume regulation protects against excessive blood loss and hemodynamic collapse. Conversely, blood volume expansion suppresses ADH regardless of plasma osmolarity. The volume threshold requires approximately 10% blood volume change before override of osmotic regulation occurs.
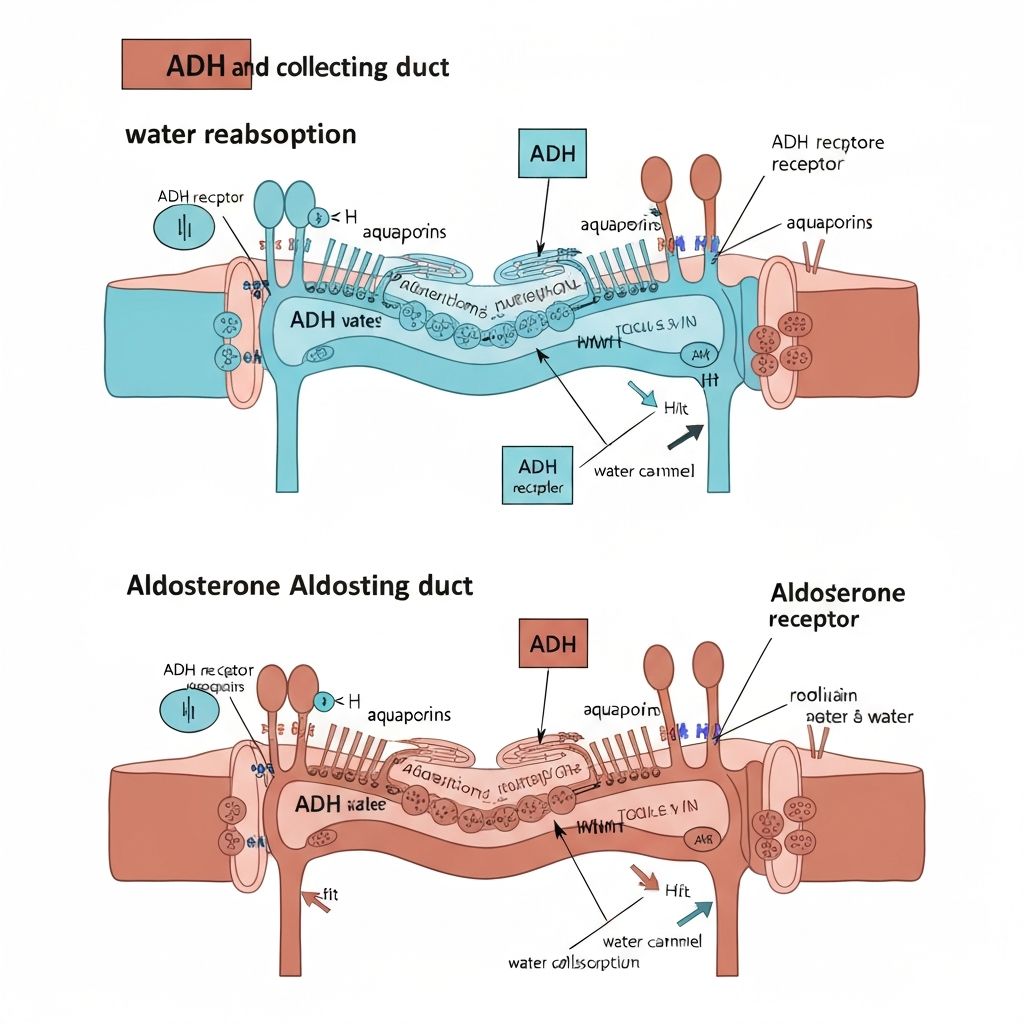
Mechanism of Action
ADH binds to V2 receptors on collecting duct principal cells. Receptor activation increases cAMP, activating protein kinase A, which phosphorylates regulatory proteins. Phosphorylated proteins insert aquaporin-2 water channels into the apical membrane, increasing water permeability. When ADH levels decline, endocytosis internalizes aquaporin-2 channels, reducing water permeability. This reversible channel regulation permits rapid adjustment of water reabsorption in response to body needs.